 |
| Perhaps having no clothes on is the real key to avoiding migraines. |
Why would the FDA be so cavalier about a device that transmits electricity into patients' skulls? Why would it accept two limited, unimpressive studies as adequate proof of "safety and effectiveness"? How can we assume that such a device can target one specific nerve --the trigeminal -- leaving adjacent nerves and brain tissues unaffected? What might be the long-term effects of using such a device on the central nervous system?
The Cefaly product is available by prescription only, the FDA said. Yet, astonishingly, this "newly approved" device has been available without a prescription at many retailers -- including Amazon.com -- for more than three years, which I learned inadvertently by doing a simple Google search. It gets 2.7 stars.
(After this article appeared, Costco and Amazon removed the product from their sites.)
Cefaly's promotional materials characterize this little battery-powered device as "innovative" and "highly effective." But if you dig up its clinical-trial data, you will find that it produced a "therapeutic gain" of just 26 percent. If your doctor prescribes Cefaly, he probably won't provide you with this information. He probably won't know anything except for what the sales rep told him ("It's a miracle!"). And he may very well not know that the FDA acknowledges "mild to moderate risk" to users of Cefaly, a "novel invention" which "is not substantially equivalent to any already legally marketed device." Are you feeling reassured?
Cefaly's promotional materials characterize this little battery-powered device as "innovative" and "highly effective." But if you dig up its clinical-trial data, you will find that it produced a "therapeutic gain" of just 26 percent. If your doctor prescribes Cefaly, he probably won't provide you with this information. He probably won't know anything except for what the sales rep told him ("It's a miracle!"). And he may very well not know that the FDA acknowledges "mild to moderate risk" to users of Cefaly, a "novel invention" which "is not substantially equivalent to any already legally marketed device." Are you feeling reassured?
 |
| "No, I'm not feeling reassured, you fucking charlatans!" |
Migraines affect nearly 30 million Americans, according to the National Headache Foundation. "Each migraine can last from four hours to three days. Occasionally, it will last longer," WebMD says.
"I am concerned about the safety of allowing brain stimulation devices
loose on the public, without prior efficacy or safety limits," said Mark
George, director of the Medical University of South Carolina's Brain
Stimulation Laboratory. "We need to proceed cautiously."
 |
| This is the picture Amazon has been using to sell the medical device. |
Something
is obviously amiss for a medical device that requires a prescription, and was
only approved in the U.S. a few days ago, to have been available without a
prescription through U.S. retailers since 2011. The "Cefaly® Anti-migraine
Device," offered by Amazon and distributed by Lynda Medical Shop, makes
claims that go well beyond the FDA approval. The product "provides a prevention
treatment program to increase the production of endorphins and raise
the trigger threshold of the pain for a result of less frequent
migraines...It blocks pain & provides relief during a migraine."
None of the italicized statements has been authorized by the FDA, and in fact
the device is not supposed to be used during a migraine. It has received one
5-star review, one 2-star and one 1-star on the Amazon site. The same photo is used by Amazon for the "Cefaly set: Anti-Migraine and Anti-stress" product. This one cleverly omits the trademark sign. Its descriptive material says:
- Frequent headaches, anxiety and stress? Cefaly can change your life
- With its pleasant stimulative effect, it inhibits pain, arrests headaches, calms anxiety and soothes stress.
- A few sessions a week are all that's needed to restore your well-being, clear your head and alleviate stress.
To quote Ricky Riccardo: "Somebody's got some 'splaining to do."
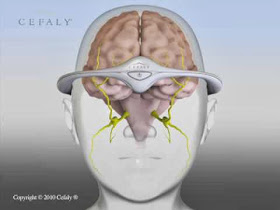 |
| The silvery plastic device purportedly targets the trigeminal nerve. |
The marketing of this product has been as credible and principled as medical-device peddling typically is -- which is to say that it's deliberately deceptive about its novelty, its therapeutic benefits and its mode of operation.
"Cefaly® is an innovative medical device protected by a series of patents. This is the first cranial external neurostimulation device on the market," the manufacturer's press release claims.
That simply is not true true. In fact, it's such a blatant and easily contradicted statement that I can't believe Cefaly made it. As I wrote in this post (http://kronstantinople.blogspot.com/2013/09/our-future-everything-in-modulation.html),
researchers around the world are developing and have developed external
neurostimulation devices to treat a wide range of conditions, including
depression, tinnitus, insomnia, anxiety, pain, and several others. I'll review these efforts at the end of this article. Here is just one of them, by California-based Neurosigma:
It is a fascinating field of study, filled with promise and peril, but it was going strong long before Cefaly got into the picture. In fact, Cefaly's TENS technology was first used in migraine research in 1980.
THE PROOF IS IN THE NOGGIN
FDA's easygoing, de novo approval of Cefaly® is based on two studies. One involved only 67 patients -- eight of whom dropped out and half of whom were in the placebo group. It resulted in a suspiciously imprecise "significant" reduction in the number (but not the intensity) of migraines. (Interestingly, the study names conflicts of interest that comprise top medical device-makers in the country -- St. Jude, Cyberonics, and Medtronic. The fourth "conflict" is Allergan, maker of Botox, which was approved in 2010 for the treatment of migraines. Its clinical trials produced a "major decrease" in the number of migraines, which sounds better than Cefaly's "significant" decrease, doesn't it?) |
| The concept is the same as Cefaly but uses a different design. |
 |
| External trigeminal nerve stimulation. |
THE PROOF IS IN THE NOGGIN
 |
| "Migraine Collage" by Migraine Chick |
 |
| Migraines cause excruciating pain in millions of Americans. |
 |
| "Migraine Headache" by Faderhead. |
THEY'VE GOT A LOT OF NERVE
Cefaly® neurostimulation is applied to the upper branch of the trigeminal nerve, which is "associated" with migraines, according to a press release from the company. "Very accurate pulses are transmitted through the electrode to the nerve endings of the trigeminal nerve."
Very accurate? How accurate can you be when you're shooting electricity into someone's head? And even if you could target the trigeminal nerve effectively, this nerve -- like most cranial nerves -- is complex, sensitive and multifunctional. What might you be doing besides (if you're lucky) preventing a migraine?
 |
| The trigeminal nerve is the largest cranial nerve. |
It has certainly not been proven that stabbing this nerve with electric currents is harmless or predictable.
"Sensory information from the face and body is processed by parallel pathways in the central nervous system: the motor division of the trigeminal nerve and the sensory division," according to Wikipedia."The trigeminal nucleus extends throughout the entire brainstem, from the midbrain to the medulla, and continues into the cervical cord, where it merges with the dorsal horn cells of the spinal cord.....The three parts of the trigeminal nucleus receive different types of sensory information."
The processing of pain/temperature information by the trigeminal nerve is very nuanced, Wikipedia notes. It all sounds very nuanced to me, and not something I'd entrust to a "nerve zapper."
 |
| Frederick Meijer Sculpture Garden in Grand Rapids / photo by MigraineChick |
" 'Pain' is a highly individualized, personal sensation that varies
markedly among different people," Wikipedia continues. "It is conditioned by their memories and
by their emotions. The fundamental anatomical differences between the
pathways for touch/position perception and pain/temperature sensation
help to explain why pain, especially chronic pain, is so difficult to
manage."
 |
| Aswirl in migraine anguish. / by fuego |
And they also help to explain why we should be somewhat cautious, don't you think, when we starting screwing around with the circuitry? I bought a TENS unit several months ago to use on my lower back. It is an "FDA cleared," nonprescription unit, which produces considerable depth of sensation with its electrical impulses. I considered applying the pads to my temples, to see if it enhanced my mood (or devastated my IQ or ability to speak), but I didn't dare. I've had electroshock treatments, which proved to me that one should not take this kind of intervention lightly, and certainly not turn it into a fashionable, futuristic adornment.
CEFALY IS NOT "THE FIRST" EXTERNAL NEUROSTIMULATION DEVICE
There are many external neurostimulators that precede the Cefaly product. For example, at the push of a button, the device shown below gives out two fleeting bursts of electricity, which short-circuit the "electrical storm" in the brain that causes the splitting pain, flashing lights and blurred vision associated with migraines, according to the manufacturer.
It has been approved in Britain by The National Institute for Health and Care Excellence, or NICE, according to media reports two months ago. "Single pulse trans-cranial magnetic stimulation is a wonderful example of clinical and laboratory research delivering a real improvement in migraine treatment that is both effective and extremely well tolerated," Professor Peter Goadsby, chair of the British Association for the Study of Headache, and director of the National Headache Centre at King's College Hospital in London, says.
There are many external neurostimulators that precede the Cefaly product. For example, at the push of a button, the device shown below gives out two fleeting bursts of electricity, which short-circuit the "electrical storm" in the brain that causes the splitting pain, flashing lights and blurred vision associated with migraines, according to the manufacturer.
It has been approved in Britain by The National Institute for Health and Care Excellence, or NICE, according to media reports two months ago. "Single pulse trans-cranial magnetic stimulation is a wonderful example of clinical and laboratory research delivering a real improvement in migraine treatment that is both effective and extremely well tolerated," Professor Peter Goadsby, chair of the British Association for the Study of Headache, and director of the National Headache Centre at King's College Hospital in London, says.
In a November, 2013 article, "Jumper Cables for the Mind," New York Times reporter Dan Hurley describes undergoing transcranial direct-current stimulation, or tDCS, an
experimental technique for delivering extremely low dose electrical
stimulation to the brain. (http://www.nytimes.com/2013/11/03/magazine/jumper-cables-for-the-mind.html?hpw).
This is another technology that preceded Cefaly's "first" external neurostimulation device.
"Using less than 1 percent of the electrical energy necessary for electroconvulsive therapy, powered by an ordinary nine-volt battery, tDCS has been shown in hundreds of studies to enhance an astonishing, seemingly implausible variety of intellectual, emotional and movement-related brain functions," he writes.
J. León Morales-Quezada, senior research associate at Harvard’s Laboratory of Neuromodulation, is conducting experiments on the ability of this therapy to improve the speed and accuracy with which people perform attention-switching tasks. It is showing promise in several areas of brain function, and has the advantage of not requiring implantation.
THE NEW "FOCUS" ON STYLE AND MIND
Electric neurostimulators have been used for years in therapy and as an adjunct to exercise, but a new device from Focus Labs claims to “increase the plasticity of your brain,” using transcranial Direct Current Stimulation (tDCS).
The $250 headset, which is not FDA-approved because it is not marketed as a medical device, sends anywhere from 0.8 to 2.0 milliamps of electrical current to your prefrontal cortex for 10 to 40 minutes. It went on the market last May. It does have physiological effects, which range from mild tingling to headaches and mood changes.
And I wrote about off-the-shelf technology that you can use on your own brain to do who-knows-what? Just mess with yourself!(http://kronstantinople.blogspot.com/2014/08/shock-treatments-can-off-shelf-brain.html)
THIS THING'S GOT GAME
"It’s mostly geared toward video gamers who want to improve their hand-eye coordination and get an edge on opponents, but if the system really does improve individual performance, perhaps one day we’ll see it used by clinical specialists like surgeons and trauma docs during procedures to improve focus and prevent mistakes. It’s controlled via Bluetooth through an iOS device like an iPad or iPhone, and you can set the strength of the current and duration for your particular needs," the Focus site explains.
Amit Etkin, an assistant professor of psychiatry and behavior
sciences at Stanford University, says the safety of
tDCS has not been verified.
"It's true that tDCS has been used in studies and even shown substantial positive effects on patients," said Etkin, whose clinic specializes in transcranial magnetic stimulation, a depression treatment that is highly regulated and poses a slight risk of seizure. But, he noted, "The worst-case scenario is that a bunch of users who don't have specific instructions will use it in a variety of different ways and alter their brains in ways that they don't anticipate and we don't anticipate."
GENTLY LIBERATING NEUROTRANSMITTERS
Then there is the Fisher Wallace Cranial Stimulator, which is aggressively marketed to treat depression, anxiety and insomnia. This device has been FDA cleared since 1991 for the treatment of insomnia, anxiety and depression, according to its web site. It works by "gently stimulating the brain's production of serotonin, beta-endorphin and other neurochemicals required for healthy mood and sleep." Like the Cefaly product, it involves external neurostimulation.
"Multiple published studies demonstrate the safety and effectiveness of the technology, which is typically used by patients for 20 minutes, twice a day (once in the morning and once before bed)," the web site says.
Research suggests that the electrical current from the Fisher Wallace device targets the limbic system, which contains brain structures linked to the experiencing of emotions, and that it stimulates the release of the feel-good neurotransmitters dopamine and serotonin. the site claims.
Chip Fisher bought the patent three years ago and now manufactures the device at his own laboratory. He said he believes that his device might be beneficial for disorders as varied as obsessive-compulsive disorder, drug addictions, attention deficit hyperactivity disorder, post-traumatic stress disorder—and, in fact, "anybody with a brain."
AND HERE WE GO AGAIN WITH THE VAGUS NERVE
Cyberonics, whose invasive, problematic VNS system has undoubtedly
deterred many potential customers -- has seen the light, and has developed an alliance with CerboMed, a private company based in Erlangen, Germany. Its NEMOS t-VNS system,
which includes an earplug-like device, is designed to stimulate a branch
of the vagus nerve in the outer ear by sending a pulse through the
skin.
CerboMed’s device has received clearance for marketing in Europe
for patients with epilepsy, depression, and pain, but is not yet approved in the U.S. In September 2013,
Cyberonics said it had made an initial investment of about $2.6 million
in Cerbomed and gained an exclusive option to market the NEMOS device
worldwide as an epilepsy treatment.
In a clinical trial in Germany, "Five of the seven patients who applied t-VNS for nine months showed a reduction in seizure frequency." This information is obviously not adequate to enable a prospective patient to make an informed decision. How much reduction? How severe was the disease in those studied?
The transcutaneous therapy is also being tested in migraine patients.
Cyberonics’ VNS system already dominates the market in devices for
epilepsy. Some 100,000 people worldwide have received its relatively
primitive and permanent implant. Think how they must feel, knowing that the wire that is gripping a nerve deep in their necks is unnecessary?
ANOTHER NONINVASIVE APPROACH FOR DEPRESSION AND EPILEPSY
Another intriguing, nonsurgical option: Los Angeles-based NeuroSigma received EU certification last year for an external trigeminal nerve stimulation (eTNS) system for the adjunctive treatment of epilepsy and major depressive disorder for adults and children aged nine years and older, according to Medscape.
The device has been evaluated in clinical trials conducted at the
University of California, Los Angeles (UCLA) and the University of
Southern California. It consists of an external pulse generator and
disposable electric patches placed on the forehead that are replaced
daily and must be worn for 12 hours a day.
But in the clinical trials, the improvement rates "did not achieve significance," according to a 2013 Medscape article. Only 30 percent of patients responded to the treatment.
The study, the first randomized, active controlled trial of external trigeminal nerve stimulation in drug-resistant partial seizures, was published in the February 26 issue of Neurology. The lead investigator was the vice president of NeuroSigma.
Commenting on the results for Medscape Medical News, Gregory Bergey, MD, from Johns Hopkins Epilepsy Center, said, "These things need more study."
An accompaning editorial stated: "The beneficial effect demonstrated by DeGiorgio et al. was modest but is
sufficient to encourage design of a more definitive study."
"As a non-invasive neuromodulation therapy, trigeminal nerve stimulation
may represent a paradigm shift in the way we treat major depression
and offers the potential to significantly improve the lives of millions
of people without the side-effects common to medication treatment,"
said UCLA's Ian A. Cook, MD, who led clinical and human mechanism of action
studies of the device in depression.
A TREATMENT WITH REAL MAGNETISM
Yet another contestant in this race to the pot of gold is Neuronetics' Neurostar mechanism, which uses a highly focused pulsed magnetic field, similar in type and strength to those produced by a magnetic resonance imaging (MRI) machine, to stimulate cortical neurons.
Another promising example of external neuromodulation comes from New Jersey-based ElectroCore, maker of the handheld gammaCore device. The firm conducted clinical trials on the use of vagus nerve stimulation to treat migraine headaches. The attractive little invention also holds promise for such disparate conditions as coma, irritable bowel syndrome, asthma and obesity, according to Scientific American.
The results of ElectroCore's trial, which involved only 21 volunteers, were presented
in July month at the International Headache Congress in Boston. Eighteen of the test subjects
reported a reduction in the severity and frequency of their headaches,
rating them, on average, 50 per cent less painful after using the device
daily and whenever they felt a headache coming on.
The device also appears to stimulate the release of inhibitory neurotransmitters, which counter the effects of glutamate, Scientific American added. This is a neurotransmitter, which is pertinent to the treatment of depression.
IN CONCLUSION:
You're not still reading this, are you? God, let's get up and give our cardiovascular systems a little workout, why don't we? I don't know about you, but my butt is totally numb. Take David Bowie's advice:
To wrap things up, I'll just say that research in the field of neuromodulation is exciting and promising on one hand, and profoundly troubling on the other. This is dangerous territory -- playing around with our brain circuitry -- and we need regulators who are committed to ensuring our health and safety. We don't have them. We have bureaucrats who are some combination of overworked, complacent and morally compromised.
Patient: Beware.
This is another technology that preceded Cefaly's "first" external neurostimulation device.
"Using less than 1 percent of the electrical energy necessary for electroconvulsive therapy, powered by an ordinary nine-volt battery, tDCS has been shown in hundreds of studies to enhance an astonishing, seemingly implausible variety of intellectual, emotional and movement-related brain functions," he writes.
J. León Morales-Quezada, senior research associate at Harvard’s Laboratory of Neuromodulation, is conducting experiments on the ability of this therapy to improve the speed and accuracy with which people perform attention-switching tasks. It is showing promise in several areas of brain function, and has the advantage of not requiring implantation.
THE NEW "FOCUS" ON STYLE AND MIND
Electric neurostimulators have been used for years in therapy and as an adjunct to exercise, but a new device from Focus Labs claims to “increase the plasticity of your brain,” using transcranial Direct Current Stimulation (tDCS).
 |
| Another stimulator with a definite "coolness" aspect. |
The $250 headset, which is not FDA-approved because it is not marketed as a medical device, sends anywhere from 0.8 to 2.0 milliamps of electrical current to your prefrontal cortex for 10 to 40 minutes. It went on the market last May. It does have physiological effects, which range from mild tingling to headaches and mood changes.
And I wrote about off-the-shelf technology that you can use on your own brain to do who-knows-what? Just mess with yourself!(http://kronstantinople.blogspot.com/2014/08/shock-treatments-can-off-shelf-brain.html)
THIS THING'S GOT GAME
"It’s mostly geared toward video gamers who want to improve their hand-eye coordination and get an edge on opponents, but if the system really does improve individual performance, perhaps one day we’ll see it used by clinical specialists like surgeons and trauma docs during procedures to improve focus and prevent mistakes. It’s controlled via Bluetooth through an iOS device like an iPad or iPhone, and you can set the strength of the current and duration for your particular needs," the Focus site explains.
 |
| Saline solution on sponges helps prevent burning of the skin. |
"It's true that tDCS has been used in studies and even shown substantial positive effects on patients," said Etkin, whose clinic specializes in transcranial magnetic stimulation, a depression treatment that is highly regulated and poses a slight risk of seizure. But, he noted, "The worst-case scenario is that a bunch of users who don't have specific instructions will use it in a variety of different ways and alter their brains in ways that they don't anticipate and we don't anticipate."
Then there is the Fisher Wallace Cranial Stimulator, which is aggressively marketed to treat depression, anxiety and insomnia. This device has been FDA cleared since 1991 for the treatment of insomnia, anxiety and depression, according to its web site. It works by "gently stimulating the brain's production of serotonin, beta-endorphin and other neurochemicals required for healthy mood and sleep." Like the Cefaly product, it involves external neurostimulation.
"Multiple published studies demonstrate the safety and effectiveness of the technology, which is typically used by patients for 20 minutes, twice a day (once in the morning and once before bed)," the web site says.
Research suggests that the electrical current from the Fisher Wallace device targets the limbic system, which contains brain structures linked to the experiencing of emotions, and that it stimulates the release of the feel-good neurotransmitters dopamine and serotonin. the site claims.
Chip Fisher bought the patent three years ago and now manufactures the device at his own laboratory. He said he believes that his device might be beneficial for disorders as varied as obsessive-compulsive disorder, drug addictions, attention deficit hyperactivity disorder, post-traumatic stress disorder—and, in fact, "anybody with a brain."
 |
| "Migraine" mosaic by Boehringer Ingelheim. |
AND HERE WE GO AGAIN WITH THE VAGUS NERVE
 |
| Isn't this a bit nicer than having a wire wrapped deep inside your neck? |
In a clinical trial in Germany, "Five of the seven patients who applied t-VNS for nine months showed a reduction in seizure frequency." This information is obviously not adequate to enable a prospective patient to make an informed decision. How much reduction? How severe was the disease in those studied?
The transcutaneous therapy is also being tested in migraine patients.
 |
| "Fragmentation" by Frederic Durville |
ANOTHER NONINVASIVE APPROACH FOR DEPRESSION AND EPILEPSY
Another intriguing, nonsurgical option: Los Angeles-based NeuroSigma received EU certification last year for an external trigeminal nerve stimulation (eTNS) system for the adjunctive treatment of epilepsy and major depressive disorder for adults and children aged nine years and older, according to Medscape.
But in the clinical trials, the improvement rates "did not achieve significance," according to a 2013 Medscape article. Only 30 percent of patients responded to the treatment.
The study, the first randomized, active controlled trial of external trigeminal nerve stimulation in drug-resistant partial seizures, was published in the February 26 issue of Neurology. The lead investigator was the vice president of NeuroSigma.
Commenting on the results for Medscape Medical News, Gregory Bergey, MD, from Johns Hopkins Epilepsy Center, said, "These things need more study."
 |
| Portrayal of epilepsy by Hieronymous Bosch. |
A TREATMENT WITH REAL MAGNETISM
Yet another contestant in this race to the pot of gold is Neuronetics' Neurostar mechanism, which uses a highly focused pulsed magnetic field, similar in type and strength to those produced by a magnetic resonance imaging (MRI) machine, to stimulate cortical neurons.
Its pulsed, MRI-strength magnetic fields induce small electric currents in the prefrontal cortex
of the brain, according to the company's website. Local neurons theoretically
depolarize and release neurotransmitters. Distant areas of the limbic
system are activated via neuronal pathways. Blood flow and glucose
metabolism rise in the stimulated regions, which is thought to result in
improved mood.
It sounds great! But they all do!
The therapy, which won the FDA nod in 2008, according to MassDevice.com, is a rarity in the med-tech world: A device-based approach to a psychiatric disorder.
Treatment is administered five days a week, for approximately four to six weeks, the firm's web site explains. The therapy "stimulates key areas of the brain that are underactive in patients with depression."
"The only other
widely used device-based therapy in psychiatry is shock therapy, and that's
been around since the 1930s," CEO Bruce Shook told MassDevice in 2011. "While the
therapy is not a cure (there is still no known "cure" for
depression), clinical tests show that patients treated with NeuroStar
experience improvement in anxiety and other symptoms associated with
depression, without side effects such as weight gain, sexual dysfunction and
nausea often associated with drug therapy."
The key word here is "improvement."
Upon further examination of the data, it was revealed that one half of patients "responded" to TMS. Half, obviously, didn't. But a "response" can mean anything from mild mood improvement to a joyful relief from gloom and anguish.
ELECTROCORE KEEPS YOU OUT OF THE OPERATING ROOM, TOO
It sounds great! But they all do!
The therapy, which won the FDA nod in 2008, according to MassDevice.com, is a rarity in the med-tech world: A device-based approach to a psychiatric disorder.
Treatment is administered five days a week, for approximately four to six weeks, the firm's web site explains. The therapy "stimulates key areas of the brain that are underactive in patients with depression."
 |
| Some brain systems seem to shut down in depressed patients. |
The key word here is "improvement."
Upon further examination of the data, it was revealed that one half of patients "responded" to TMS. Half, obviously, didn't. But a "response" can mean anything from mild mood improvement to a joyful relief from gloom and anguish.
ELECTROCORE KEEPS YOU OUT OF THE OPERATING ROOM, TOO
Another promising example of external neuromodulation comes from New Jersey-based ElectroCore, maker of the handheld gammaCore device. The firm conducted clinical trials on the use of vagus nerve stimulation to treat migraine headaches. The attractive little invention also holds promise for such disparate conditions as coma, irritable bowel syndrome, asthma and obesity, according to Scientific American.
 |
| ElectroCore's device treats migraines and is being tested for tinnitus. |
The device also appears to stimulate the release of inhibitory neurotransmitters, which counter the effects of glutamate, Scientific American added. This is a neurotransmitter, which is pertinent to the treatment of depression.
IN CONCLUSION:
You're not still reading this, are you? God, let's get up and give our cardiovascular systems a little workout, why don't we? I don't know about you, but my butt is totally numb. Take David Bowie's advice:
Put on your red shoes and dance the blues
Under the moonlight. The serious moonlight.
The moonlight needs to be serious these days. Everybody with any power is screwing everything up. Poor moon. She used to be radiantly cheerful.To wrap things up, I'll just say that research in the field of neuromodulation is exciting and promising on one hand, and profoundly troubling on the other. This is dangerous territory -- playing around with our brain circuitry -- and we need regulators who are committed to ensuring our health and safety. We don't have them. We have bureaucrats who are some combination of overworked, complacent and morally compromised.
Patient: Beware.






